Justin Heinonen, Jeremy Wilson and A-CAPP Center Staff, 2010
The Need for Data on Product Counterfeiting
Despite the staggering economic, health and safety effects of product counterfeiting, there are few evidence‐based lessons on the problem. A major reason there is so little systematic research on product counterfeiting is that reliable data are typically non‐existent or restricted. To address this problem, Michigan State University’s Anti‐Counterfeiting and Product Protection Program (A‐CAPPP) is building the first‐ever national open‐source product counterfeiting incident database.
Theory and Practice Guide the Database
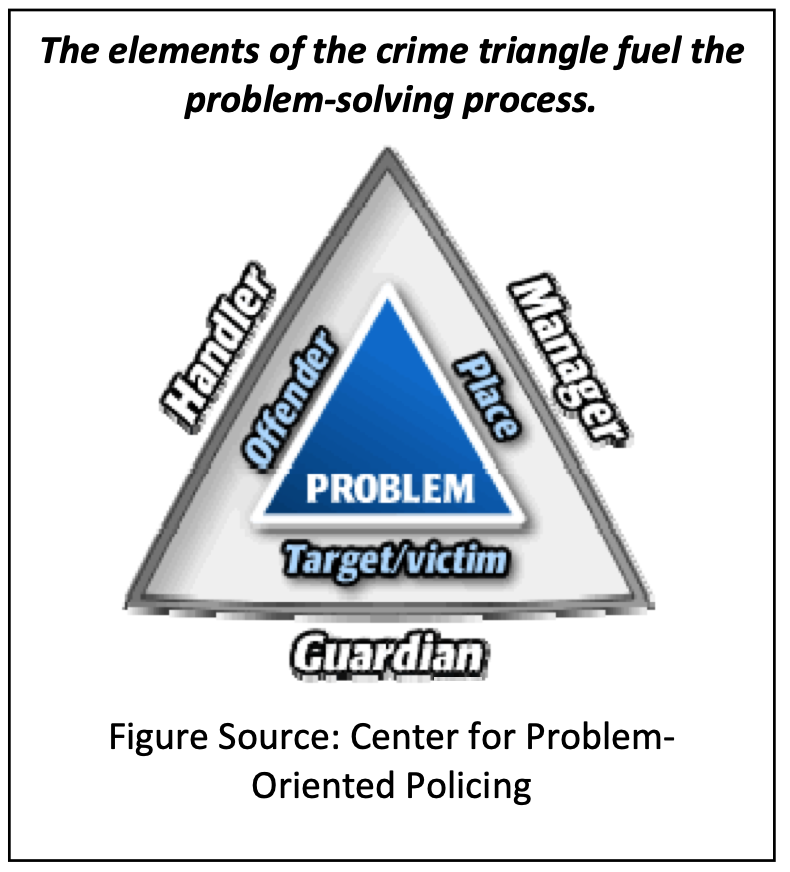
The database is built upon the tenets of problem‐solving, the crime analysis triangle, and situational crime prevention. Policymakers can effectively address crime when the characteristics and processes underlying it are identified and examined. Our database captures information about product‐counterfeiting suspects, victims, and places, as well as the handlers, guardians, and managers that interact with them. Analyzing such data increases our understanding of the problem and can reveal intervention points for tailoring effective prevention strategies. Such strategies could increase the risk and effort, reduce the rewards and provocations, and remove excuses associated with product counterfeiting.
Our Method is Systematic and Thorough
We use a systematic process to identify U.S. incidents of product counterfeiting and to gather and code open‐source information related to them. We have used this method to build a database specific to pharmaceutical counterfeiting. We ultimately hope to expand our scope to compile an ongoing comprehensive database covering all products, industries, and regions around the globe. We have already identified and can leverage hundreds of incidents related to counterfeiting of luxury goods and apparel, electronics, food and beverages, transportation components and other manufactured goods.
Step 1: Identifying Incidents
To identify all incidents of product counterfeiting in general, we searched the websites of various government agencies and industry organizations that monitor product counterfeiting. We reviewed from these websites all reports, case studies, press releases, speeches and other documents related to product counterfeiting. We also conducted keyword searches at these websites as well as online databases and news outlets. To date, we have reviewed more than 3,100 publications from various media, agency, industry and scholarly sources from which we have identified from all industry sectors an initial list of over 800 unique, publicly reported incidents of product counterfeiting.
Step 2: Gathering Open‐Source Information
Next, we developed two web‐based search engines to capture as much open‐source information as possible for the more than 100 incidents of pharmaceutical counterfeiting identified in step one. Trained “searchers” enter keywords from each incident and search simultaneously seven web‐engines grouped within a “primary” protocol and then thirteen web‐engines within a “secondary” protocol. Primary and secondary searches uncover various published open‐source material, including media accounts, government documents, court records, videos, blogs and online forums, books, watch‐group reports, and scholarly accounts. Searchers scan these materials and record all relevant information for each incident in a standard file.
Step 3: Coding Information
Finally, we are continually revising formal instruments to code the open‐source information in each file. This will permit us to create unique databases on suspects, victims, incidents, and types of drugs counterfeited. The open‐source information is useful for describing characteristics and processes associated with the chemistry of these crimes. It establishes the basic parameters of the problem (e.g., the number of and attributes of suspects and the quantification of harm in terms of seized goods and victims) as well as the response to it (e.g., which agencies identify and investigate the offenses and for what the suspects are charged, convicted, or sentenced). Once the data are completely coded, we will be able to provide empirical insights into more complex features of pharmaceutical counterfeiting.
The Database Advances Research and Policy
The incident database provides an opportunity for continued empirical investigation into the problem of product counterfeiting. A‐CAPPP researchers will be able to reveal new insights about elements that are instrumental to understanding and responding to U.S. product counterfeiting. Lessons derived from this work will aid the problem‐solving process and thereby better inform decision‐making among policymakers and practitioners regarding the prevention, detection, investigation, and intervention of product counterfeiting in general and in specific industries. To be sure, generalizing from the database has limitations in that it only captures incidents that have somehow come to the public’s attention. Nevertheless, it serves as a rich, empirical foundation where none yet exists and captures information about cases that are arguably the most important and for which concerted intervention is needed
2010 Copyright Michigan State University Board of Trustees.